|
 What is pH?: The terms acid
and base describe chemical characteristics
of many substances that we use daily. We use bases to make soaps
and detergents, for example. Almost all the foods we eat, from bread
to coffee, are slightly acidic.
What is pH?: The terms acid
and base describe chemical characteristics
of many substances that we use daily. We use bases to make soaps
and detergents, for example. Almost all the foods we eat, from bread
to coffee, are slightly acidic.
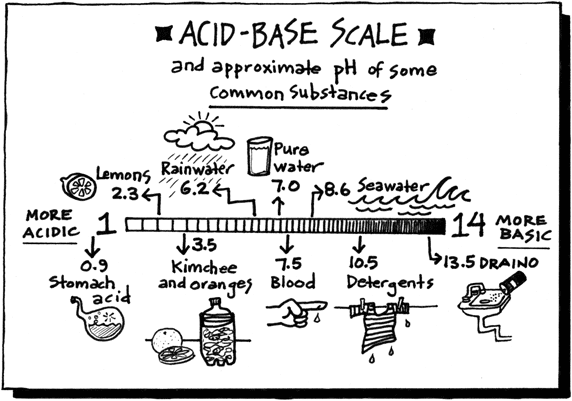
The pH scale is a measure of acidity. You can buy pH indicator
paper from any biological or lab supply company, which can be used
to give you an accurate measurement of the acidic or basic quality
of substances you want to test. You can also make your own pH indicator
using red cabbage juice, for example, to track the changing pH in
your fermentation chamber.
Make your own acid/base indicator: Blend
2 cups chopped red cabbage leaves and 1 cup water in a food processor
or electric blender until pieces are tiny and uniform. Strain the
liquid. You can also chop the cabbage coarsely and boil it in the
water for about 5 minutes until the liquid is dark purple. This
purple liquid will change color according to the acidity or alkalinity
(basic quality) of substances you want to test.
 Add
about 10 drops of cabbage juice to approximately 1 tablespoon of
a test substance. What color does cabbage juice turn in an acid
like white vinegar? What about in a base such as a baking soda solution? Add
about 10 drops of cabbage juice to approximately 1 tablespoon of
a test substance. What color does cabbage juice turn in an acid
like white vinegar? What about in a base such as a baking soda solution?
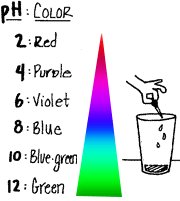
Test the pH of various substances and develop a corresponding color-pH
scale.Compare your results with the chart here.
You can also make indicator paper by
dipping strips of white paper towel, coffee filters, or construction
paper into the cabbage juice until they are purple. When the purple
strips are dry, use a toothpick, soda straw or eye dropper to place
a drop of a test solution on the strips. How do the results compare
to your pH chart?
What does salt do?
Osmosis and density changes in kimchee
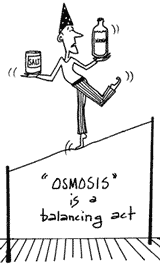 Osmosis:
When you sprinkle salt on cabbage leaves and then exert pressure
on them, you'll notice that the leaves become limp and the bottle
begins to fill with liquid. Just what is going on here? Osmosis:
When you sprinkle salt on cabbage leaves and then exert pressure
on them, you'll notice that the leaves become limp and the bottle
begins to fill with liquid. Just what is going on here?
Liquid inside the cells of the cabbage leaves is flowing out of
the cells in response to the salt, a phenomenon called osmosis.
Osmosis is the movement of fluid through a membrane in order to
create an equal concentration of dissolved solids (salt, in this
case) in the fluid on either side of the membrane. When you salt
a cabbage leaf, you create a difference in salt concentration —
higher outside the cabbage leaf and lower inside the cells. In order
to equalize the salt concentration, water from inside the cabbage
leaf cells will exit through the cell membranes. The water will
continue to flow out of the cells until there is an equal concentration
of salt in the fluids on either side of the cell wall, or until
the cell is completely dehydrated.
 The
2% solution: People who pickle often use a 2% salt concentration.
How much salt must you add to your 2-liter bottle of cabbage to
produce a 2% salt concentration? The
2% solution: People who pickle often use a 2% salt concentration.
How much salt must you add to your 2-liter bottle of cabbage to
produce a 2% salt concentration?
It just so happens that a 2-liter soda bottle packed full of cabbage
leaves weighs approximately 1 kilogram. Chinese cabbage is about
95% water, so you need only to figure out how much salt you need
to add to 1 kilogram (1,000 grams) of water in order to create a
2% solution. Two percent of 1,000 (.02 x 1,000) is 20 — you
need 20 grams of salt. Since one 35 mm film can holds 40 grams of
salt, a film can half full of salt is a good measure of what you
will need.
Density changes: During the first few
hours after you fill your kimchee bottle, you will observe that
the height of cabbage leaves falls by half. The weight of the bottle
remains the same, but the density changes. How can you quantify
the density changes in your 2-liter bottle?
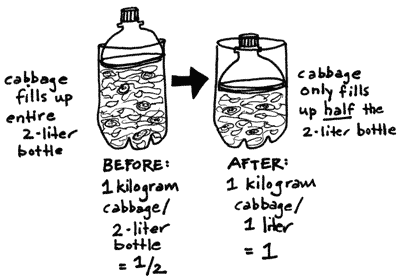
A 2-liter bottle newly packed full of leaves weighs about a kilogram.
Since density is grams per volume (d=g/v), your kimchee or sauerkraut
has an initial density of 1 kilogram of cabbage per 2-liter soda
bottle, or 1/2.
Due to osmosis, however, the cabbage level drops to about half
the bottle. In other words, the volume drops to 1 liter. Now the
density equals 1 kilogram per 1 liter, or 1. The mass of the bottle
hasn't changed, but the density has doubled.
|

